Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a well-established therapeutic modality for a variety of hematological malignancies and congenital disorders. One of the major complications of the procedure is graft-versus-host-disease (GVHD) initiated by T cells co-administered with the graft. Removal of donor T cells from the graft is a widely employed and effective strategy to prevent GVHD, although its impact on post-transplant immune reconstitution might significantly affect anti-tumor and anti-infectious responses. Several approaches of T cell depletion (TCD) exist, including in vivo depletion using anti-thymocyte globulin (ATG) and/or post-transplant cyclophosphamide (PTCy) as well as in vitro manipulation of the graft. In this work, we analyzed the impact of different T cell depletion strategies on immune reconstitution after allogeneic HSCT.
Methods
We retrospectively analysed data from 168 patients transplanted between 2015 and 2019 at Geneva University Hospitals. In our center, several methods for TCD are being used, alone or in combination: 1) In vivo T cell depletion using ATG (ATG-Thymoglobulin 7.5 mg/kg or ATG-Fresenius 25 mg/kg); 2) in vitro partial T cell depletion (pTCD) of the graft obtained through in vitro incubation with alemtuzumab (Campath [Genzyme Corporation, Cambridge, MA]), washed before infusion and administered at day 0, followed on day +1 by an add-back of unmanipulated grafts containing about 100 × 106/kg donor T cells. The procedure is followed by donor lymphocyte infusions at incremental doses starting with 1 × 106 CD3/kg at 3 months to all patients who had received pTCD grafts with RIC in the absence of GVHD; 3) post-transplant cyclophosphamide (PTCy; 50 mg/kg) on days 3 and 4 post-HSCT. Absolute counts of CD3, CD4, CD8, CD19 and NK cells measured by flow cytometry during the first year after allogeneic HSCT were analyzed. Measures obtained from patients with mixed donor chimerism or after therapeutic DLI were excluded from the analysis. Cell numbers during time were compared using mixed-effects linear models depending on the TCD. Multivariable analysis was performed taking into account the impact of clinical factors differing between patients groups (patient's age, donor type and conditioning).
Results
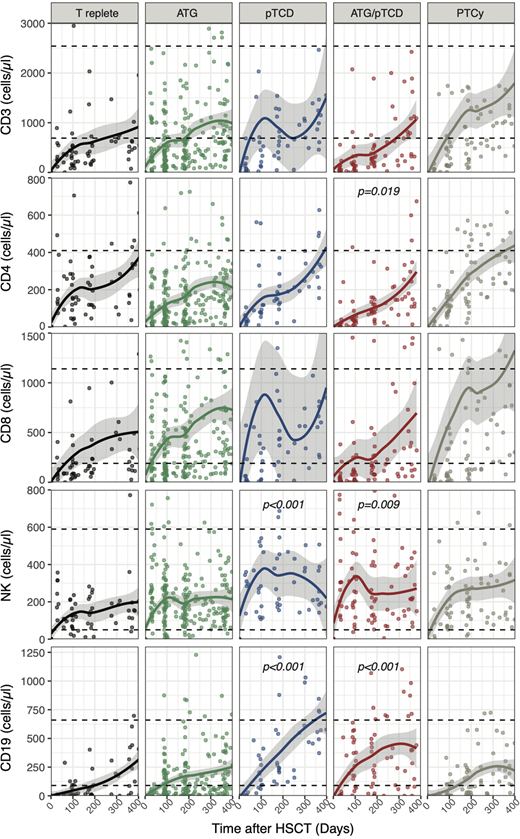
ATG was administered to 77 (46%) patients, 15 (9%) patients received a pTCD graft and 26 (15%) patients received a combination of both ATG and pTCD graft. 24 (14%) patients were treated with PTCy and 26 (15%) patients received a T replete graft. 60% of patients had a reduced intensity conditioning (RIC). 48 (29%) patients received grafts from a sibling identical donor, 94 (56%) from a matched unrelated donor, 13 (8%) from mismatched unrelated donor and 13 (8%) received haploidentical grafts. TCD protocols had no significant impact on CD3 or CD8 T cell reconstitution during the first year post-HSCT (Figure 1). Conversely, CD4 T cells recovery was affected by the ATG/pTCD combination (coefficient ± SE: -67±28, p=0.019) when compared to the T cell replete group (Figure 1). Analysis of data censored for acute or chronic GVHD requiring treatment or relapse revealed a delay of CD4 T cell reconstitution in the ATG and/or pTCD treated groups on (ATG:-79±27, p=0.004; pTCD:-100±43, p=0.022; ATG/pTCD:-110±33, p<0.001). Interestingly, pTCD alone or in combination with ATG resulted in a better reconstitution of NK cells compared to T replete group (pTCD: 152±45, p<0.001; ATG/pTCD: 94±36, p=0.009; Figure 1). A similar effect of pTCD was also observed for B cells (pTCD: 170±48, p<.001; ATG/pTCD: 127±38, p<.001). The effect of pTCD on NK was confirmed when data were censored for GVHD and relapse (pTCD: 132±60, p=0.028; ATG/pTCD: 106±47, p=0.023) while only ATG/pTCD retained a significant impact on B cells (102±49, p=0.037). The use of PTCy did not affect T, NK or B cell reconstitution when compared to the T cell replete group.
Conclusion
Our results indicate that all TCD protocols with the only exception of PTCy are associated with a delayed recovery of CD4 T cells whereas pTCD of the graft, alone or in combination with ATG, significantly improves NK and B cell reconstitution.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.